Fundamental and applied research comes together via international networking, using the facilities of the CESGA Super-Computer Center (Spain), the Brazilian Synchrotron Light Laboratory (LNLS), and the special synthesis technique of subnanometer-sized metal clusters by the company NANOGAP: de Lara-Castells et al. [1] address the question how a special surface treatment can change the optical properties of TiO2. This compound is a semiconductor material which is known to many as a bright white pigment used in numerous applications ranging from paint over sunscreens and even toothpaste. In skincare products, titanium dioxide is often used due to its ability to adsorb UV light, the high-energy part of the solar spectrum.
Fundamental and applied research comes together via international networking, using the facilities of the CESGA Super-Computer Center (Spain), the Brazilian Synchrotron Light Laboratory (LNLS), and the special synthesis technique of subnanometer-sized metal clusters by the company NANOGAP: de Lara-Castells et al. [1] address the question how a special surface treatment can change the optical properties of TiO2. This compound is a semiconductor material which is known to many as a bright white pigment used in numerous applications ranging from paint over sunscreens and even toothpaste. In skincare products, titanium dioxide is often used due to its ability to adsorb UV light, the high-energy part of the solar spectrum.
Now, these excellent adsorption properties triggered the following thoughts: Can this adsorption be somehow improved even further?. Can one take advantage of this semiconductor to somehow harvest more of the energy the sun provides, and is there a chance to directly steer this energy into chemical reactions?.
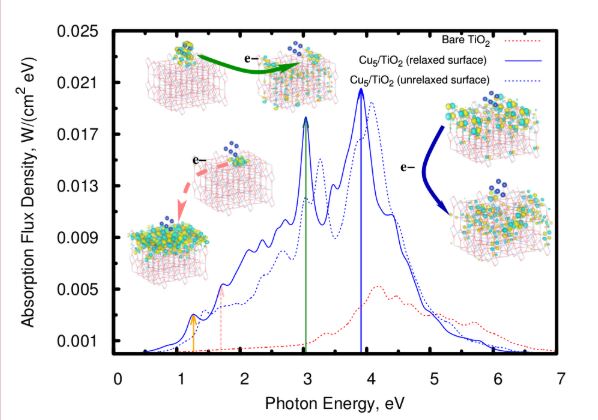
In order to answer these questions, the authors [1] propose the employment of tiniest copper nanoparticles, smallest metal atomic clusters of sub-nanometer size. The authors demonstrate that, when deposited on the surface of titanium dioxide, the copper clusters are able to shift the adsorption from the high energy range, i.e. the UV spectrum, towards the visible light, where the sun has its maximum energy output. As a consequence, much more energy can be harvested from sunlight, and the coated titanium dioxide stores this energy temporarily in the form of charge pairs – electrons and holes – which is a perfect prerequisite for follow-up chemistry.
This is a big step forward towards harvesting solar energy for follow-up surface reactions, and work is in progress to link this outcome to some relevant photocatalysis in the next step. Mimicking photosynthesis processes, triggered by sunlight, these novel photocatalysts offer new strategies to eliminate CO2 and other common pollutants in the atmosphere through oxidation processes activated by solar energy.
Dr. Maria Pilar de Lara-Castells (CSIC, AbinitSim group) tells us how the work was performed and how the computational resources of CESGA were used: “I would highlight that all the computational resources employed were provided by CESGA and that the work is a very illustrative case of the predictive capacity of ab-initio multiscale modelling of novel nanostructured materials, having been the theoretical results validated by the experimental measurements. I also consider the work as a clear product of the networking activity within the COST CM1405 action “Molecules in Motion”. In fact, within this context, the collaboration of my group with both the University of Santiago of Compostela and NANOGAP and the University of Technology of Graz emerged.”
Source
[1]: “Increasing the optical response of TiO2 and extending it into the visible through surface activation with highly stable Cu5 clusters” M. P. de Lara-Castells, A. W. Hauser, J. M. Ramallo-López, D. Buceta, L. J. Giovanetti, M. A. López-Quintela, F. G. Requejo; Journal of Materials Chemistry A 7 (2019) 7489. DOI: 10.1039/C9TA00994A